Knee Replacement
KNEE PROBLEMS
SURGICAL PROCEDURES
Your New Knee
One of the most important orthopaedic surgical advances of this century,
knee replacement was first performed in 1968. Improvements in surgical
materials and techniques since then have greatly increased its effectiveness.
Total knee replacement means resurfacing the bones of your knee joint
with a 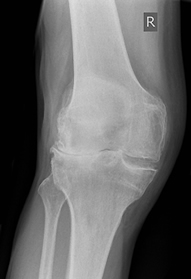 prosthesis.
prosthesis.
Of the three surfaces in your knee that may become roughened and painful, you may need two or all three surfaces replaced. Like a normal knee, your implants have smooth weight-bearing surfaces. The femoral component covers your thighbone, the tibial component covers the top of your shinbone, and the patellar component (if used) covers the underside of your kneecap. All components are usually cemented to prepared bone surfaces.
Total knee replacement is as safe and reliable as total hip surgery, although your knee is a more complex and less stable joint than your hip. The studies of modern knee arthroplasty report clinical survivorship of up to 96% of total knee implants at 10 to 15 years. The average survivorship of total knees is over 12 years.
Indications
Indications for knee replacement surgery have expanded during the last decade. During the 1970s and 1980s, knee replacement surgery was performed mainly for pain, disability or deformity. The expected benefit from the operation was reduced pain, limb realignment and functional improvement. However, pain was the primary reason for knee replacement surgery. As we enter a new century, function is a primary reason for knee replacement surgery. Patients are not satisfied with the reduced function that can accompany a stiff, painful arthritic knee and they are demanding knee replacement operations to improve their function. Increasingly, the desire for functional improvement includes recreational and sports activities.
Benefits of a Knee Replacement
Once your new joint has completely healed you should experience following benefits of the surgery: significantly reduced or no joint pain, increased movement and mobility, correction of angular leg deformity, increased leg strength (if you exercise!), improved quality of life and the ability to return to most normal activities and pastimes.
Implants
We have been using DePuy PFC Sigma knee replacement implants since 1996, with good results and without implant complications and failures, so far.
PFC Sigma knee system is the result of more than 30 years evolution in total knee replacement. It has developed into a universal system, with solutions for almost any indication. The components are designed to match the demands of today's patients for high function and low wear. More than 200,000 PFC knees have been implanted at some 800 centres worldwide.

Johnson & Johnson DePuy PFC Sigma Knee System
Implanted in the UK since 1998 in 347 hospitals by more than 2900 surgeons in the public and independent sectors, the Sigma knee system continues to deliver excellent results for patients and surgeons. With more than 77,000 implanted in five years the Sigma knee system is the most frequently used implant in the UK National Joint Registry. From five year NJR data no other top 10 knee implants had lower revision rates than Sigma.
ConforMIS Personalised Knee Implants
We are pleased to announce that we will start using ConforMIS iTotal and iUni patient specific knee implants from September 2014. Mr Vladimir Bobic introduced this new technology to the Grosvenor Hospital Chester in 2013. After almost two years of preparations, regulatory and clinical negotiations with healthcare insurers and Nuffield Health, imaging and theatre staff training we are finally ready to go!
ConforMIS team has developed the only personalized knee resurfacing implants available today, designed to conform precisely to individual patient's unique anatomy. Using a proprietary technology called iFit (the "i" stands for individualized), ConforMIS creates implants which are made specifically for each patient. Personalized implants offer unique advantages versus traditional knee replacement options: superior fit matched to individual size and shape, natural, anatomic feel and alignment, significant bone preservation, less traumatic procedure and potentially faster recovery. For many patients, especially those who suffer from earlier stages of osteoarthritis, ConforMIS offers a range of implants that may provide a less invasive, less traumatic option than traditional knee replacement.


ConforMIS iTotal and iDuo Personalised Knee Replacement Implants
The iFit image-to-implant process uses CT imaging data to create personalized devices designed to achieve precise fit, preserve bone and cartilage, and minimize surgical trauma. This process begins when the patient receives a diagnostic CT scan. After the scan, the centre sends the data to ConforMIS via CD or secure internet file transfer. Using iFit technology, ConforMIS generates a 3D model of the patient's knee and then map the anatomy of the patient's joint. This technology creates an implant that is precisely matched to the patient's surface contours and anatomy.
For more information on knee replacement surgery please visit: www.kneereplacement.com
What Materials are Used for Implants?
Joint implant manufacturers, orthopaedic surgeons and scientists continually strive to improve the durability of these devices. Current scientific advances in metallurgy have resulted in the use of titanium and cobalt-chrome alloys, which are used for femoral and tibial implants, and a low friction plastic component, made of ultra-high molecular weight polyethylene polymer, which acts as a spacer and articulating surface between two metal components. All materials used in moving surfaces are very durable, but they will eventually wear out. This is as true in surfaces of artificial joint implants as in those of car tires. Orthopaedic companies have been working hard to find better materials that will not wear out for a long time.
 |
 |
|
Johnson & Johnson DePuy PFC
Sigma knee implants |
||
Does Patella Need Replacing?
The jury is still out and patellar resurfacing in knee arthroplasty remains controversial. Numerous clinical trials have been done to help clarify the indications for patellar resurfacing. Unfortunately, there is little consensus, and surgeon preference remains the primary variable. Despite the numerous trials, there are three basic strategies: always resurface, never resurface, or selectively resurface the patella.
We do not replace patellae routinely, except in patients with severe patella deformities and rheumatoid arthritis.
Further information on patella resurfacing:
-
Bonin N, Mercado J, Deschamps G, DeJour D: Resurfacing versus not resurfacing the patella in total knee arthroplasty: 4 year results. Presented at the 13th ESSKA 2000 Congress. May 21-24, 2008. Porto, Portugal. A review of 85% of the patients undergoing resurfacing and 80% of the control group revealed no significant differences regarding patient satisfaction, International Knee Society (IKS) revision scores and clinical anterior knee pain scores at 4-year follow-up. Source: Gina Brockenbrough: Similar results seen in TKA patients with resurfaced vs. native patellae at 4 years. Orthopedics Today Europe, November 2008; 11:9.
-
Emilios E. Pakos, et al.: Patellar Resurfacing in Total Knee Arthroplasty. A Meta-Analysis. The Journal of Bone and Joint Surgery (American). 2005;87:1438-1445.
-
R. Stephen Burnett and Robert B. Bourne: Indications for Patellar Resurfacing in Total Knee Arthroplasty. AAOS Instructional Course Lecture. The Journal of Bone and Joint Surgery (American). 2003;85:728-745.
-
Robert L Barrack, et al.: Resurfacing of the Patella in Total Knee Arthroplasty. A Prospective, Randomized, Double-Blind Study. The Journal of Bone and Joint Surgery (American). 1997;79:1121-1131.
Obesity and a Knee Replacement
There has been considerable discussion as to the influence of obesity on the indications for, and the outcome after, knee replacement. Although this is logistically, anaesthetically and surgically more difficult procedure, with additional risks involved, the functional outcomes of knee replacements in obese people appear to be satisfactory.
However, attempts have been made in the east of England to withhold NHS funding for such procedures in those who are overweight. The editorial published in the British edition of the Journal of Bone and Joint Surgery examined the current evidence concerning the influence of obesity on joint replacement and it appears that it is only in the morbidly obese, with a body mass index (BMI) > 40 kg/m2, that significant contraindications to operation are present. The Editor concludes: "Overall, although there have been no controlled trials which have assessed the influence of obesity, the current evidence suggests that there is no statistically significant difference in outcome in hip and knee replacement as influenced by weight unless the patient is morbidly obese, when the results begin to worsen. However, in these patients the improvement in their quality of life is still considerable and, provided they have been made aware of the increased risks, operation should not be withheld." Source:
- F. Horan, Editor Emeritus: Obesity and joint replacement. Editorial, The Journal of Bone and Joint Surgery - British Volume, October 2006, 88-B (10) 1269-1271.
The research, published online in the journal Annals of the Rheumatic Diseases, shows that clinically obese people (those who have a body mass index of above 30 kg/m2) can benefit almost as much as anyone else from the procedure. Source:
- Medical News Today: Research Indicates No Justification For Denying Obese Patients Knee Replacements. Online First Ann Rheum Dis 2008.
Equally, there is substantial evidence of significantly increased risk of complications after lower limb joint surgery in patients who are morbidly obese and increasing evidence that obese patients too have a potentially increased risk of complications. Furthermore, evidence of implant failure after surgery in the morbidly obese, and increasing evidence of reduced implant survival in the obese population suggest that not only are there immediate dangers associated with surgery in this group of patients, but limitations with regard to longer term efficacy and durability. The clearly established links between obesity and other disorders such as cardiac disease and diabetes mellitus make it important for medical counselling prior to any surgery, and increasingly there is evidence that obesity itself should be discussed as not only an indirect, but a direct risk for complications and compromised results. Source:
- G.N. Gillespie, A.J. Porteous: Obesity and knee arthroplasty. The Knee 14 (2007) 81–86.
Sports after a Knee Replacement
Our duty is to educate patients regarding risks associated with higher levels of activity after total knee replacement: implant loosening, accelerated wear of the articulating surfaces and injuries. We advise our patients to avoid recreational and athletic activities until their quadriceps and hamstring muscles are sufficiently rehabilitated. After muscle strength has been recovered, we try to help our patients to make reasonable choices regarding athletic activities. In general, we support and encourage low-impact activities: cycling, golf, dancing, riding, walking (but not jogging), swimming and tennis (but not squash). Please see The Knee Society recommendations for more information:
-
Recommended activities : cycling is an excellent aerobic workout. Calisthenics, swimming, low-resistance rowing, stationary skiing machines, walking, hiking, and low-resistance weight lifting all are excellent ways to maintain fitness without overstressing the implant. Suitable activities include bowling, croquet, golf, doubles tennis, table tennis, ballroom dancing and square dancing. Other activities that are suitable but slightly more risky include downhill skiing, scuba diving, in-line skating, ice skating, softball, volleyball, speed walking, horseback riding, hunting and low-impact aerobics.
-
Discouraged activities: in general, patients who have undergone total knee replacement should avoid high-impact activities that cause high stress loads on the implant and therefore may increase the risk of early failure. Activities to avoid include baseball, basketball, football, hockey, soccer, high-impact aerobics, gymnastics, jogging, power lifting , rock climbing, hang gliding, and parachuting.
-
For further, more activity-specific information, please see the Knee Society Survey table and the JBJS article below the table:
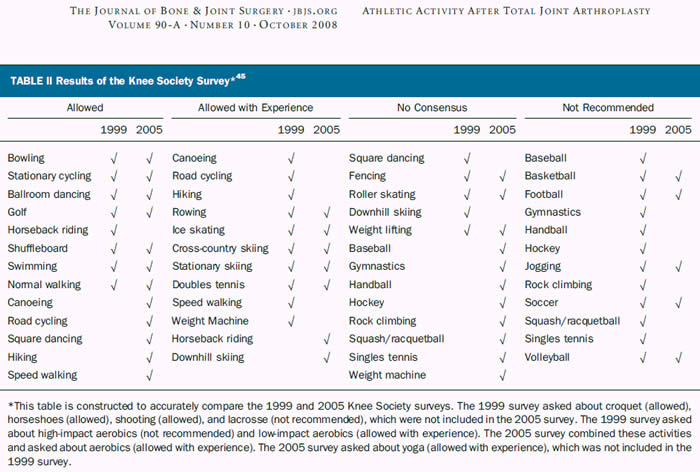
Orthopaedic articles on athletic activities after knee replacement:
The orthopaedic literature on athletic activity after total joint arthroplasty is limited to small retrospective studies with short-term follow-up:
- Healy WL, et al.: Athletic Activity After Total Joint Arthroplasty. The Journal of Bone and Joint Surgery (American). 2008;90:2245-2252.
- Mont MA, et al.: Tennis after Total Knee Arthroplasty. The American Journal of Sports Medicine 30:163-166 (2002).
Further information on athletic activities after knee replacement:
- Activities after Knee Replacement: After You Leave the Hospital. KneeReplacement.com, 13 November 2006.
- Activities After a Knee Replacement. AAOS , www.orthoinfo.org.
Complications After a Knee Replacement
Knee replacement surgery is generally very successful, and complications are relatively uncommon, considering the complexity of the procedure. However, complications can occur following a knee replacement, as with all major surgical procedures. They include excessive swelling or bleeding, blood clots (DVT or deep vein thrombosis), pulmonary embolism (PE), phlebitis, neurovascular damage, skin healing problems, subcutaneous stitch abscess, peri- and intra-articular infection, limited flexion or extension or both, stiff joint (arthrofibrosis), early loosening of implants, allergy to the metal parts of the implants, fracture of the knee bones, etc. There are also anaesthetic risks, both during and after the procedure.
Further information:
- Sibanda N, et al., on behalf of the Steering Committee of the National Joint Registry (NJR) for England and Wales: Revision Rates after Primary Hip and Knee Replacement in England between 2003 and 2006. PLoS Medicine, September 2, 2008. Summary: According to the results of a British retrospective review of 80,697 patients with a primary total knee arthroplasty (TKA) , 1 in 75 patients needed revision surgery within 3 years. Three-year revision rates for TKA patients were 1.4% for cemented prostheses, 1.5% for cementless prostheses, and 2.8% for unicondylar prostheses. Patients younger than age 55 at the time of the primary TKA had the highest revision rate; those older than age 75 had the lowest revision rate. (AAOS Headline News Now, 8 October 2008).
VTE (Venous Thromboembolism) Prevention:
Although there is no doubt that a knee replacement, like many other major orthopaedic surgical procedures, is associated with increased risk of venous thromboembolism the jury is still out as to what are the best preventative measures. Please see the following document for further information:
- NICE Clinical Guideline 46. Venous thromboembolism: reducing the risk of a blood clot from surgery. Information for people who use NHS services, April 2007.
- Considering that the above guidelines are generic, the following update was issued by NICE:
When Can I Drive After a Knee Replacement?
You may be able to drive 2 to 4 weeks after your operation, or even earlier, depending on your general health, mobility, the range of movement of your replaced knee and muscle strength. It is a good idea to practice moving your feet on the pedals while the car is stationary. This will give you a good idea of how you will be able to manage. You should be able to do an emergency stop before attempting to drive. You should discuss the timing of going back to driving with your surgeon and your physiotherapist. For further information please visit DVLA’s website (www.dvla.gov.uk).
The following recent clinical study adds further evidence-based information on this issue: "Clinical studies of brake response time have suggested that patients should be advised to wait 30 days postoperatively before resuming driving if they had a right TKA and 10 days if they had a left TKA, as long as they drive a car with automatic transmission and not under the influence of narcotics. In our study, we allowed patients to self-determine when to resume driving by suggesting that they wait to drive until they felt safe behind the wheel, so that if they got in an accident it was not because they had difficulties driving the car but because of a judgment error. We observed that by 4 weeks, 48% of patients (85 of 177) with a right TKA and 57% of patients (74 of 129) with a left TKA resumed driving. When patients considering TKA ask when they can expect to drive, we remind them that they should only drive when they feel safe behind the wheel and that they will drive sooner if they have their left knee replaced and drive a car with an automatic transmission than if they have their right knee replaced or drive a car with a manual transmission." Stephen M. Howell, MD and Stephanie L. Rogers, MPT. Method for Quantifying Patient Expectations and Early Recovery After Total Knee Arthroplasty. ORTHOPEDICS 2009; 32:884.
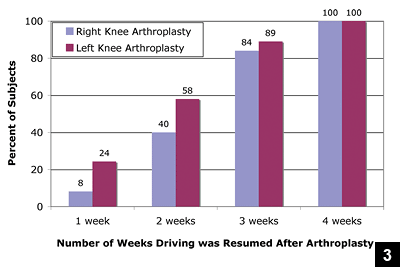 |
Column graph showing the distribution of the percentage of patients who resumed driving at 1, 2, 3, and 4 weeks. By 4 weeks, 48% of patients (85 of 177) with a right TKA and 57% of patients (74 of 129) with a left TKA resumed driving. At 2 weeks, a post-hoc analysis showed that a higher percentage of patients with a left TKA returned to driving than with a right TKA (steering wheel on right side of vehicle). Table source: Stephen M. Howell, MD; Stephanie L. Rogers, MPT. Method for Quantifying Patient Expectations and Early Recovery After Total Knee Arthroplasty. ORTHOPEDICS 2009; 32:884. |
When Can I Fly After a Knee Replacement?
There is no universal agreement as to when it is safe to travel by plane after a knee replacement. It seems that most orthopaedic surgeons advise their patients not to fly for at least 4 to 6 weeks before and after a knee replacement. Although short flights do not seem to be a problem long intercontinental flights are a potential problem as there is an increased incidence of spontaneous DVT (deep venous thrombosis), even in the young and healthy passengers. It is possible that sitting for long period of time, in a confined space and with very little leg room, could predispose to the development of deep venous blood clots, especially in people following recent major knee surgery. The likelihood of developing postoperative leg blood clots depends on many different factors, including your general health, medical history, postoperative mobility and a number of risk factors (obesity, smoking, a history of DVT, etc.). If you have to travel by plane, before 4 weeks after your knee replacement, it would be wise to contact your airline’s Medical Department and to ask them for advice. Also, please discuss this issue with your GP, as you should take further prophylactic measures for several weeks.
General advice on travel-related DVT prophylaxis:
- Department of Health. Advice on travel-related DVT. DoH website, last modified on 12 March 2007.
- Mike J Clarke, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database of Systematic Reviews (Intervention Review), Issue 4, 2008 (re-published online with edits on 8 October 2008).
- People Logistics. When Can I fly? People Logistics Limited website. January 8, 2007.
Dental Treatment After a Knee Replacement
The routine administration of prophylactic antibiotics for dental interventions to prevent haematogenous spread of infections to prosthetic joints is a contentious issue. There is a lack of robust evidence linking dental procedures to an increased risk of infection of prosthetic joints. Antibiotic prophylaxis has not been shown to effectively prevent dental-associated infections. Basically, there is no evidence to link prosthetic joint infections to dental procedures and none to prove that antibiotic prophylaxis is effective. Source:
-
T F Oswald and F K Gould: Dental treatment and prosthetic joints. ANTIBIOTICS ARE NOT THE ANSWER! Editorial. Journal of Bone and Joint Surgery (British). 2008; 90-B: 825-826.
-
I Uçkay, et al.: Antibiotic prophylaxis before invasive dental procedures in patients with arthroplasties of the hip and knee. Journal of Bone and Joint Surgery (British). 2008; 90-B: 833-838.
Downloads:
- Chester Knee Clinic Total Knee Replacement Patient Brochure

- Chester Knee Clinic Total Knee Replacement Rehabilitation
Guide

- OSUMC Guidelines For Knee Rehabilitation

- Aircast Knee Cryo/Cuff gravity cooler: cooling and compression postoperative device

Further Information:
-
MHRA Medical Device Alert: Knee replacement implant - PFC Sigma cruciate retaining non-porous size 5 left femoral component (part number 960005) manufactured by DePuy International Limited. Lot numbers of affected devices, which were manufactured from 20th January to 30th April 2009 inclusive, are listed here.
Page last updated on 13 May 2018
Site last updated on: 28 March 2014
|
[ back to top ]

 Disclaimer: This website is a source of information
and education resource for health professionals and individuals
with knee problems. Neither Chester Knee Clinic nor Vladimir Bobic
make any warranties or guarantees that the information contained
herein is accurate or complete, and are not responsible for
any errors or omissions therein, or for the results obtained from
the use of such information. Users of this information are encouraged
to confirm the accuracy and applicability thereof with other sources.
Not all knee conditions and treatment modalities are described
on this website. The opinions and methods of diagnosis and treatment
change inevitably and rapidly as new information becomes available,
and therefore the information in this website does not necessarily
represent the most current thoughts or methods. The content of
this website is provided for information only and is not intended
to be used for diagnosis or treatment or as a substitute for consultation
with your own doctor or a specialist. Email
addresses supplied are provided for basic enquiries and should
not be used for urgent or emergency requests, treatment of any
knee injuries or conditions or to transmit confidential or medical
information. If you have sustained a knee injury or have a medical condition,
you should promptly seek appropriate medical advice from your local
doctor. Any opinions or information,
unless otherwise stated, are those of Vladimir Bobic, and in no
way claim to represent the views of any other medical professionals
or institutions, including Nuffield Health and Spire Hospitals. Chester
Knee Clinic will not be liable for any direct, indirect,
consequential, special, exemplary, or other damages, loss or injury
to persons which may occur by the user's reliance on any statements,
information or advice contained in this website. Chester Knee Clinic is
not responsible for the content of external websites.
Disclaimer: This website is a source of information
and education resource for health professionals and individuals
with knee problems. Neither Chester Knee Clinic nor Vladimir Bobic
make any warranties or guarantees that the information contained
herein is accurate or complete, and are not responsible for
any errors or omissions therein, or for the results obtained from
the use of such information. Users of this information are encouraged
to confirm the accuracy and applicability thereof with other sources.
Not all knee conditions and treatment modalities are described
on this website. The opinions and methods of diagnosis and treatment
change inevitably and rapidly as new information becomes available,
and therefore the information in this website does not necessarily
represent the most current thoughts or methods. The content of
this website is provided for information only and is not intended
to be used for diagnosis or treatment or as a substitute for consultation
with your own doctor or a specialist. Email
addresses supplied are provided for basic enquiries and should
not be used for urgent or emergency requests, treatment of any
knee injuries or conditions or to transmit confidential or medical
information. If you have sustained a knee injury or have a medical condition,
you should promptly seek appropriate medical advice from your local
doctor. Any opinions or information,
unless otherwise stated, are those of Vladimir Bobic, and in no
way claim to represent the views of any other medical professionals
or institutions, including Nuffield Health and Spire Hospitals. Chester
Knee Clinic will not be liable for any direct, indirect,
consequential, special, exemplary, or other damages, loss or injury
to persons which may occur by the user's reliance on any statements,
information or advice contained in this website. Chester Knee Clinic is
not responsible for the content of external websites.